Connie: Good evening, dear friends. It’s great to have you here with us. Thank you for joining us. Today, we invite Professor Hofstadter to our program. Thanks for being here, Dr. Hofstadter.
Dr. Hofstadter: Thanks for having me. I’m glad to be here.
Connie: The microorganisms present in the intestinal tract, also known as the gut microbe, are the main internal environment members of the human body and can be used as one of the important environmental factors affecting health. Gut microbiota plays an extremely important role in the metabolism of nutrients, the body’s development, immunity, and disease production. Studies have shown that changes in the structure and function of the gut microbiota are closely related to diabetic phenotypes such as hyperglycemia and insulin resistance. So today, we are going to discuss the importance of gut microbes on diabetes mellitus management. But before we get into the effect of probiotics, can you first introduce us to diabetes, Dr. Hofstadter?
Dr. Hofstadter: Sure. Diabetes is a metabolic disorder characterized by impaired blood glucose levels. To be more specific, it’s a chronic disease caused by an inherited or acquired deficiency in the production of insulin by the pancreas or the inability of the body to make adequate use of the insulin produced. The affected individuals must routinely manage their lifestyle. Currently, diabetes is prevalent among 10 percent of adults worldwide. The number of cases is predicted to be 592 million with 175 prediabetic patients by 2035, according to the international diabetes federation.
Connie: From what I learned before, diabetes can be classified into two categories. Type 1 diabetes mellitus is an autoimmune disease triggered by the devastation of insulin-releasing beta cells in the pancreas, leading to absolute insulin deficiency. But type 2 diabetes mellitus is a metabolic disorder characterized by a progressive loss of insulin secretion from pancreatic beta cells, low insulin release, insulin resistance, and inefficiency of the body to utilize insulin.
Dr. Hofstadter: That’s right. To be more specific, type 2 diabetes mellitus is a multifactorial disease. Patients have decreased sensitivity or increased resistance to insulin, which further leads to various metabolic changes, such as increased free fatty acids, protein kinase C activation, oxidative stress, endothelial dysfunction, and inflammation. In addition, reduced incretin hormone such as glucagon-like peptide-1 secretion as well as an increased cluster of extra counter-regulatory hormones, including Dipeptidyl peptidase-4, alpha, beta glucosidases, also contribute to diabetes.
Connie: So you mean the main difference between type 1 diabetes mellitus and type 2 diabetes mellitus is whether they are insulin dependent. Also, type 1 diabetes mellitus is an immune disease, and type 2 diabetes mellitus is more of metabolic disease. But regardless of what categorizes diabetes, I guess what we all really want to know is how we can prevent them?
Dr. Hofstadter: Unfortunately, the cause of type 1 diabetes mellitus is still unknown and cannot be prevented with current knowledge. However, it is commonly accepted that obesity is one of the most important triggers for type 2 diabetes development. Therefore, reducing weight and maintaining weight through exercise can prevent the development of type 2 diabetes mellitus.
Connie: I see. Can you talk about the traditional treatment? You know, like the insulin injection.
Dr. Hofstadter: Yeah. One goal of insulin therapy for type 1 diabetes mellitus is to ensure that patients have a good quality of life, and what I mean by quality of life is that patients can avoid severe hypoglycemia as much as possible. The other goal of insulin therapy is to satisfactorily control the metabolic level, or actively prevent diabetic complications. Older patients can also use metformin and glucosidase inhibitor drugs. But for type 2 diabetes, the treatment is a bit different. The combined usage of insulin and hypoglycemic drugs is usually used. Common hypoglycemic drugs include biguanides, sulfonylureas, thiazolidinedione, dipeptidyl peptidase 4 receptor inhibitors, and sodium-dependent glucose transporters 2 receptor inhibitors, and glucagon-like peptide 1 analogs.
Connie: By now, most of us know that an unhealthy diet can increase the mortality rate of Gram-negative bacteria, leading to the development of lipopolysaccharides in the intestine, which can then be transferred into intestinal capillaries as well as in general circulation. Then reactive oxygen species generated during the subsequent oxidation process will reduce the development of mucus in the intestinal epithelium and cause injury to the epithelial cell membranes leading to increased permeability of intestinal tight junctions. So my question is, What happens to the gut microbiota of patients with diabetes?
Dr. Hofstadter: So before getting into the answer to this question, we need to first be clear that the intestinal mucosa is a major site for pathogen invasion. When undamaged, it provides the first line of defense against antigens. The intestinal wall is constituted of a layer of mucus, immunoglobulin A secreting cells, antimicrobial peptides, and a complex system of the epithelial barrier formed by adhesion and tight junctions. Individuals susceptible to type 1 diabetes and other autoimmune diseases present inadequately functioning intestinal barriers, which greatly expose antigens to the immune system.
Connie: Maybe we should talk about it more specifically, because I suppose that the changes of the gut microbiota as a result of diabetes would not be that easy.
Dr. Hofstadter: That’s right. Actually, for type 1 diabetes mellitus, the relationship between diabetes diagnosis and microbiota changes is not fully understood. What we know so far is that people with type 1 diabetes mellitus have fewer types of microbes in the microbial community. Certain types of bacteria are more common in people with type 1 diabetes mellitus than people without type 1 diabetes mellitus. This usually means fewer beneficial microorganisms. The drastic changes in the gut microbiota may increase the possibility of inflammation in patients with type 1 diabetes mellitus. Like you mentioned earlier, you know that type 1 diabetes mellitus is partly caused by inflammation of pancreatic cells that secrete insulin. Inflammation occurs when something destroys your body cells and the immune system releases chemicals that increase blood flow and support to the area.
Connie: What about type 2 diabetes mellitus?
Dr. Hofstadter: Well. Similar to type 1 diabetes, for type 2 diabetes mellitus, there are many new studies in this field, but many questions still don’t have clear answers. Type 2 diabetes mellitus may be linked to the composition of the intestinal microbiota and is directly responsible for the induction of low-grade inflammation. Additionally, the composition of the intestinal microbiota plays a significant role in the development of pre-diabetic conditions, such as insulin resistance. Some studies showed that type 2 diabetes mellitus have fewer butyric acid bacteria and an increased number of Lactobacilli. The application of the gastrointestinal metagenomic model can predict type 2 diabetes mellitus-related phenotypes, suggesting that the gut microbiota may become a new biomarker for predicting type 2 diabetes mellitus. Researchers found that glycosylated hemoglobin and blood glucose levels in patients with type 2 diabetes mellitus were positively correlated with the abundance of Lactobacillus, and negatively correlated with the abundance of Clostridium.
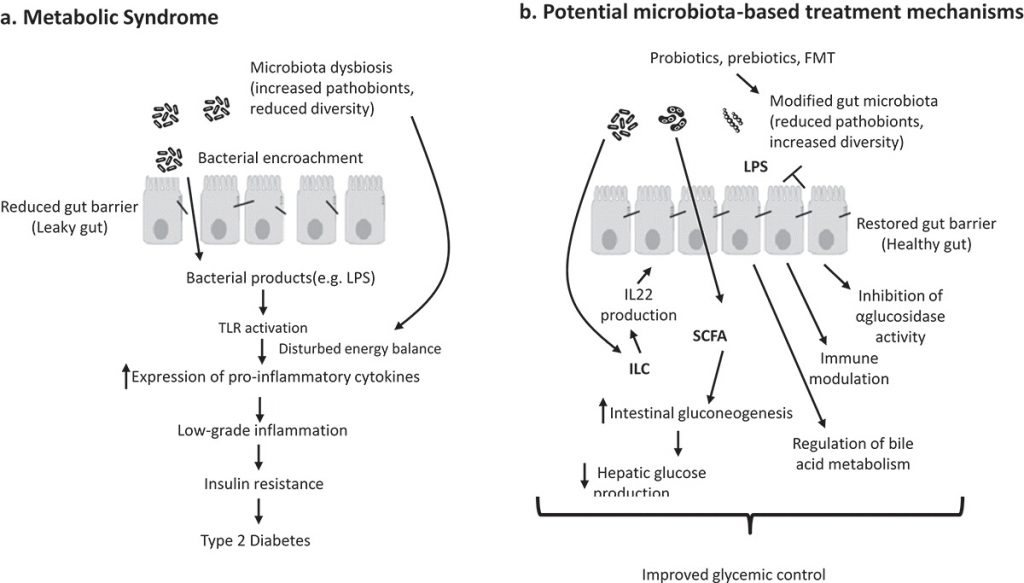
Connie: And I also found that some studies have suggested that probiotics, as good intervention options in metabolic diseases, can positively alter intestinal microbiota safely and effectively and as a consequence a positive response in diseases. In general, probiotics have shown beneficial effects, and various mechanisms have been proposed for diabetes mellitus therapy.
Dr. Hofstadter: That’s right. Now we can talk about the mechanism of action of probiotics. It’s related to the positive properties of various cellular components, secreted proteins, carbon-based acids, bacteriocins, and fatty acids. First of all, probiotics can produce metabolites that serve to increase the supply of nutrients used through intestinal epithelial cells. In addition, probiotics enhance the permeability of the intestine and prevent the passage of lipopolysaccharide through systematic circulation, thus declining metabolic endotoxemia.
Connie: I just wanted to add that Probiotics can also express microorganism-associated molecular patterns, which may bind to host pattern recognition receptors situated on the intestinal epithelial cells cell surface also dendritic cells. First, probiotics stimulate dendritic cells that inhibit the pro-inflammatory proliferation of CD4 positive cells and trigger anti-inflammatory pathways and the proliferation of plasma cells, which can result in the development of anti-inflammatory cytokines and immunoglobulin A. Let’s move on and discuss the anti-diabetic efficiency of probiotic strains investigated in vitro and in vivo.
Dr. Hofstadter: Sure. Speaking of in vitro and in vivo studies on probiotics, there have been many studies conducted to investigate their great health benefits to improving immune system function and preventing diseases such as dysentery, cancer, allergic, and human immunodeficiency virus diseases. Probiotics have also been shown to decrease blood glucose by enhancing inflammation and preventing beta-cell death in animal models. This is closely related to our topic today. It had been found in a recent analysis that L. Plantarum improves the anti-diabetic activity of high fructose ingestion of insulin receptor substrate 1, protein kinase B and endothelial nitric oxide synthase reduced renal protein expression in rat and partial supplements of L. helveticus.
Connie: Another interesting research showed that Lactobacillus acidophilus, L fermentum, L gasseri, and L rhamnosus modulate the expression of genes encoding junction and adhesion proteins such as E-cadherin and beta catenin. These strains can reduce the expression of protein kinase C delta. And just so you know, the activation of this kinase can result in the dispersion of adherence junctions, which will increase intestinal permeability.
Dr. Hofstadter: Have you heard of dahi? It’s a kind of traditional Indian fermented milk. It contains L. acidophilus, L. casei, and L. lactis. And its consumption can reduce the glycemic curve and glycated hemoglobin |glycosylated hemoglobin. And there is L. Plantarum, which is also suggested to reduce glycemia, improve glucose tolerance and reduce insulin resistance.
Connie: Now that you mentioned dahi, it reminded me of an interesting clinical study using yogurt to manage patients with type 2 diabetes. The probiotic and conventional yogurts containing Lactobacillus delbrueckii subspecies bulgaricus and Streptococcus thermophilus enriched with Bifidobacterium animalis subspecies lactis Bb12 and Lactobacillus acidophilus strain La5 were administered at 300 grams per day for 8 weeks to these patients. Patient serum analysis showed a substantial reduction in levels of glycated hemoglobin glycosylated hemoglobin besides inflammatory marker tumor necrosis factor-alpha. Researchers also observed a non-significant reduction in interleukin 6 and hypersensitive C reactive protein levels in the intervention set of serum relative to the control group. They have also done meta-analysis studies, which showed that mixed probiotic administration to rats for a minimum of 8 weeks significantly reduced weighted mean difference and improved glucose metabolism.
Dr. Hofstadter: Very interesting. I’ve heard about the outcome of probiotics on the regulation of hypertension and dyslipidemia on blood compression and the lipid contour of type 2 diabetic patients. Probiotics expressively decreased blood levels of triglyceride and total cholesterol and low-density lipoprotein cholesterol. Despite their impact on increased high-density lipoprotein cholesterol was not statistically significant on patient body mass index and age. And probiotic fermented milk was observed to diminish blood glucose and glycated hemoglobin glycosylated hemoglobin levels in the fasting blood. And serum total cholesterol levels were reduced, although not statistically significant. These results indicate that probiotic fermented milk may help to control the medical nutrition of diabetic patients.
Connie: It is now well established that the benefits of probiotics include protection from vast varieties of illnesses including infectious diseases, immunological dysfunctions, neurodegenerative disease, and metabolic disorders. The efficacy of probiotics for human health promotion is backed with an ample amount of scientific evidence and they are practiced by medical professionals as dietary, preventive, or supplementary interventions for the treatment of several diseases. In this regard, we can say that probiotics hold a new ray of hope for the betterment of human health. So much for our content today. Thanks, everyone, for listening. I hope you enjoyed today’s discussion. Thank you, Professor Hofstadter, for your time and wonderful input. We will continue our discussion next week. See you then.
